Each month I will cover two research articles on nutrition, training, sleep, supplements, or anything else that might help you. If you’d like me to cover a specific study next month send me a message on Instagram.
*Note from Brandon: if you want to learn how to interpret research go read each study before reading the breakdown below, take notes, then compare your interpretation to mine.
The two studies I’m going to cover this month are a bit older, but they answer questions that we get all the time here at TCM. Time to jump back a decade or so to get some answers.
Study #1
Title: The effects of ibuprofen on muscle hypertrophy, strength, and soreness during resistance training
Big Question: Is it ok to take NSAIDs after your workout?
Scientific Question: Do NSAIDs inhibit muscle strength and hypertrophy gains?
Why
Non-steroidal anti-inflammatory drugs (NSAIDs) are one of the most commonly used medications. In fact, athletes use NSAIDs to mitigate pain and recovery more than anything else. The general population takes them too, which is why you should care what they’re doing to your body composition and performance. We use NSAIDs for preventing pain, continuing training in spite of injuries, or shortening the time needed to get back to training after an injury. One RCT demonstrated that NSAIDs enabled more patients to return to normal after an ankle sprain than a placebo. Yet, a recent meta-analysis found NSAIDs could not reduce time to exhaustion, or self-perceived pain, although there was a trend toward a reduction of pain in athletes. Another meta-analysis suggests that they don’t alleviate DOMS better than a placebo. Taken together, the evidence on NSAIDs actually reducing pain is mixed. There are possible detriments to taking NSAIDs too.
The current study is based on the idea that high doses of ibuprofen can inhibit muscle protein synthesis (MPS) after a session of resistance training. If we inhibit MPS it could interfere with our long-term goals of gaining muscle. The thing about MPS measures is that it is an acute measure, and may not represent the long-term response well. Thus, to truly know if they prevent muscle gain we need a study that lasts a few weeks with a training program that is practical. That’s exactly what this study did. The author’s goal was to determine the effect of a moderate dose of ibuprofen (400 mg?/day) consumed on a daily basis after resistance training on muscle hypertrophy and strength.
Who
18 subjects (12 males, 6 females) participated in the study. They were 24 years old on average. They had a mean weight of ~73.6kg and were experienced lifters. Untrained participants were not included because the researchers wanted to focus on the muscular adaptation, not the neural adaptation that occurs in a short-term training study.
Study Details
A counter-balanced, double-blinded design was used. Double-blind means that participants nor the researchers knew which drug (or placebo) they were taking. Participants were randomly assigned to take ibuprofen (2×200 mg tablets per day) immediately after training the biceps of one arm and placebo after training the opposite arm the next day. Using a within-subject design, where both arms exercise, allows each participant to act as his or her own control.
Participants trained five days a week for 6 weeks.
For training, six sets were performed per session: 3 sets of 8-10 curls at 70% 1RM and 3 sets of 4–6 eccentric repetitions at 100% of 1RM. Participants had 1-minute rest between sets. Eccentric contractions were controlled at 3 seconds per repetition. Dumbbell weights were used so that the prescribed number of repetitions would result in training until failure on each set.
Progressive overload was used. Muscle thickness and strength were determined before and after the 6 weeks of training and muscle soreness was evaluated each day during the training program.
Results
After six weeks of training, both groups increased muscle strength; however, there was no difference between placebo or ibuprofen. Both groups increased strength by ~25%.
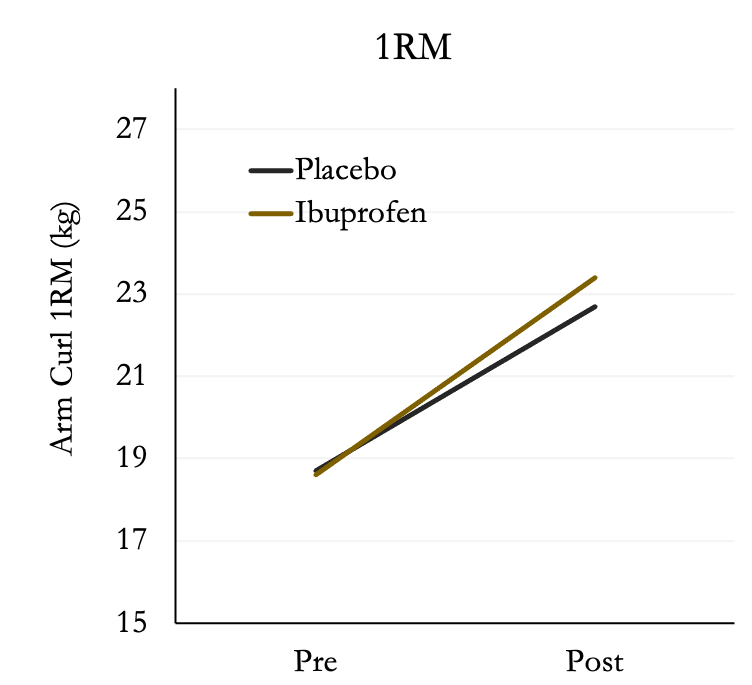
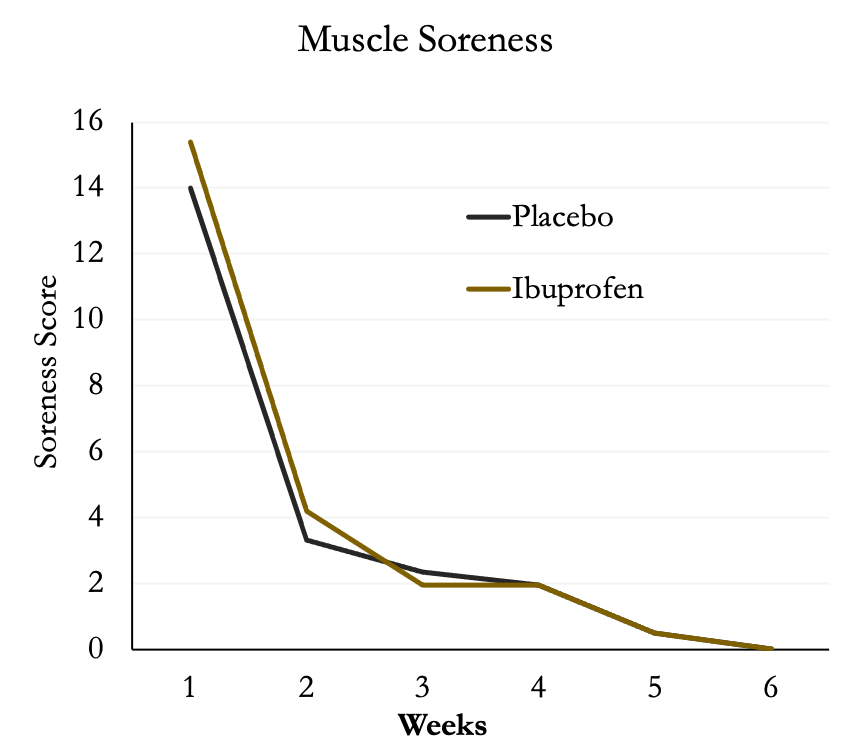
Surprisingly, muscle soreness was not different between the two groups and decreased substantially after the first week of training then leveled off.
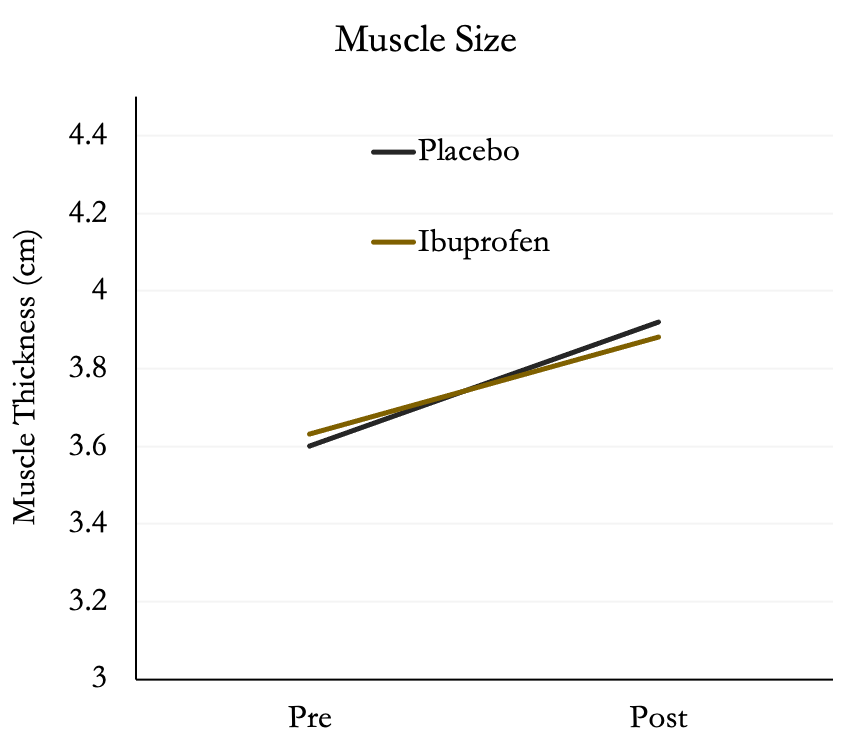
The authors also found no differences between groups in muscle size in the biceps. Both groups increased muscle size by ~8%. We wouldn’t expect a lot of change in size since the participants were trained.
Author’s Answer
“This study was the first to investigate chronic daily ibuprofen consumption over the course of a resistance training program. Contrary to our hypothesis, ibuprofen had no effect on muscle hypertrophy and strength.”
My Answer
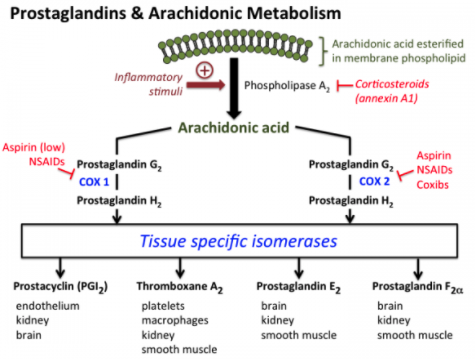
NSAIDs may alter the acute and chronic response to resistance training because they inhibit the cyclooxygenase (COX) enzymes that regulate prostaglandin synthesis. Prostaglandins, including the key molecules PGF2a and PGE2, are biological mediators of inflammation and pain. You can see from the diagram the general pathways involved.
You’ll notice that the COX enzymes act on arachidonic acid. If that sounds familiar you may be thinking of when arachidonic acid was touted as a potential supplement to promote hypertrophy made popular by William Llewellyn. Research suggests it may augment training-related adaptations by lessening the inflammatory response in muscle although the evidence is mixed.
Moving on to NSAIDs.
In the early 2000s, a study was published showing that a large dose of ibuprofen (1200mg) can inhibit MPS following high-intensity eccentric exercise. This finding set in motion a sequence that hasn’t stopped yet. Like Newton’s cradle.
Over the next few years, more evidence emerged. In 2010, Burd et al., gave a 600mg dose of celecoxib (an NSAID) to healthy young adults after 10 sets of 10 repetitions of eccentric-only knee extension at 120% of 1RM. They found no change in MPS compared to a placebo.
A year later, in 2011, Mikkelsen et al., gave healthy young participants who were endurance-trained a 45mg dose of indomethacin (another NSAID) before, during, and after exercises consisting of 200 unilateral knee extensions. They also found no differences in either MPS between the NSAID-infused leg compared with placebo at 24–28 hours post-exercise.
If you’re reading these exercise protocols and thinking “wtf” you’re not alone. People don’t do muscle damage protocols as normal training. The idea is that if NSAIDs played a role in these extreme protocols then they may also play a role in less extreme training. From the above, you can see that NSAIDs don’t really affect MPS. So, do they play a role anywhere else? There’s evidence they do.
Satellite cells are important for adaptation to training. They sit on the outside of muscle cells and fuse into muscle when damage or growth occurs to provide more myonuclei. This allows us to keep growing or recover properly. There is some evidence that the exercise-induced increase in satellite cells is prevented when taking NSAIDs meaning that long-term NSAID use could result in maladaptation. The two studies mentioned above are short term, which led to a few long-term (ish) studies.
The current study by Krentz et al., found that a moderate daily ibuprofen intake (400mg) during six weeks of training the biceps did not influence the strength or hypertrophy response, and did not help muscle soreness. A more recent study using a higher dose (1200mg) found that ibuprofen reduces strength and muscle hypertrophy adaptations to 8 weeks of resistance training in young adults.
Based on current evidence, there is little reason to believe that the occasional use of moderate doses of NSAIDs will negatively affect muscle growth or strength gains. What’s more interesting is they may not even help with pain or discomfort.
How can we apply this?
Think twice before reaching for NSAIDs for muscle pain for two reasons:
- They may not actually improve your feeling of soreness
- They may lower long-term training adaptations if taken in large doses (1200mg/day).
What’s next?
We still need a few more studies to determine at what point NSAIDs are detrimental to muscle growth. For example, if we take them for a few days at a moderate dose (800mg) does it influence our training adaptations? There is also some data to indicate they play a role in bone health.
Study #2
Title: Adipocyte turnover: relevance to human adipose tissue morphology
Big Question: Do obese people have more fat cells or bigger fat cells?
Scientific Question: Does adipocyte turnover involve different morphologies of subcutaneous adipose tissue?
Why
Fat cells have three main functions: lipid storage, secretory function, and insulin sensitivity. We have two main options when we store excess energy: we can either make fat cells larger (adipocyte hypertrophy) or we can make more of them (adipocyte hyperplasia). The question is which option is better and can we even control it?
Larger fat cells correlate with insulin resistance and an increased risk of developing type 2 diabetes. That’s not good. Adipocyte hypertrophy – unlike muscle hypertrophy – may also cause inflammation and negatively influence our metabolism. Again, not good. The problem is that we can’t specifically tell our adipocytes to get bigger or to multiply.
The mechanisms responsible for the development of different forms of adipose hypertrophy and hyperplasia are unknown. However, adipocyte creation and death may be involved. The turn-over rate of adipocytes results in about one-tenth of the total fat cell pool being renewed every year by ongoing adipocyte creation and death. If you’re thinking you could use this to your advantage you’re not alone. The current study tried to determine if adipocyte turnover was involved in the different morphologies of subcutaneous adipose tissue. Morphology means simply the shape or size of cells.
Who
In a cross-sectional design, 207 men and 557 women were recruited. They were between the ages of 18-77. 38% of them were men and 57% of them women.
In a subset of 35 participants, the relationship between adipose tissue morphology and adipocyte turnover was determined. This subset of participants was part of a different study.
Study Details
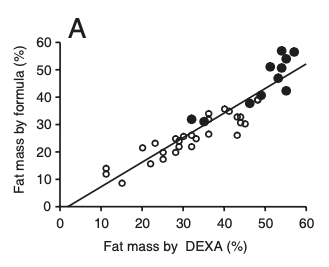
Total body fat was determined by a formula based on age, sex, and BMI. In 555 of the subjects, body fat was also determined by using bioelectrical impedance.
Adipocytes were taken via biopsies from all participants. The researchers isolated fat cells and the diameter of 100 cells was measured, then the volume and weight were calculated with a formula.
The total number of fat cells in the body was determined by dividing total body fat content (measured by the first formula) with the mean adipocyte weight (measured by the second formula). I’m not going to
put the formulas here because they are way beyond scope of a research review. Instead, I’ll show you a graph from their paper that demonstrates the first formula for body fat was very well correlated with DEXA measurements.
The relation between adipose tissue morphology and adipocyte turnover was determined in 35 participants who were not part of the larger study. This measure involves infusing a heavy carbon and measuring the turnover rate. It’s pretty difficult.
The study of adipocyte turnover was done on a separate group of subjects from a previous study. The turnover data were re-calculated and set in relation to adipose tissue morphology. These studies required very large amounts of adipose tissue (>100 g) and were taken from people who had liposuction.
The authors also created a morphology value. It was the difference between measured and expected adipocyte size given by the curved-line fit for a given body mass. A positive value means that adipocyte was larger than expected, and a negative value meant they were smaller than expected.
That’s a lot of details and you can see how they pulled data from a number of different studies to create this paper.
Results
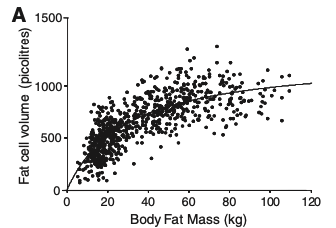
The relationship between fat mass and adipocyte size fits a curve-linear model, which means that adipose tissue development is due to a combination of an increase in the size of preexisting adipocytes and the generation of new adipocytes. If people with more fat only had larger fat cell volume it would be a directly linear curve. If people only had hyperplasia it would be very flat (in volume) and they would have more fat cells. It’s a clear mix of the two based on the graph.
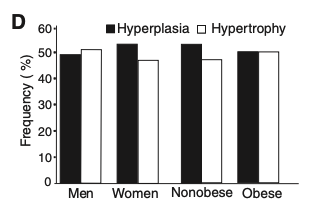
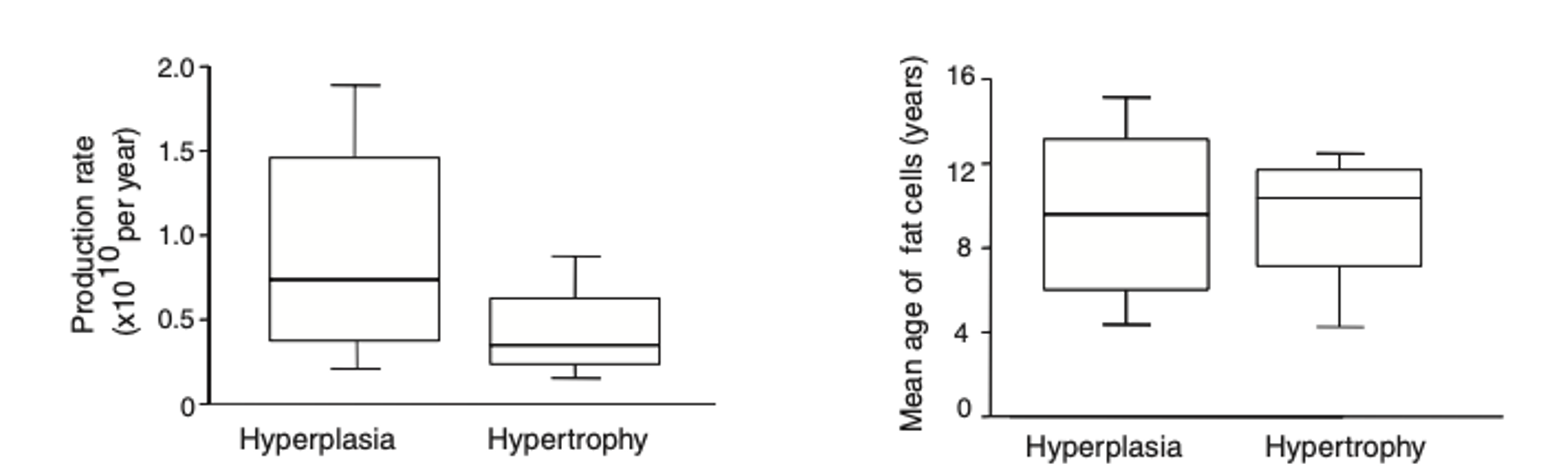
The authors also found, in the sub-study, that age, production rate, and relative death rate (?10% per year) of adipocytes were not different between hyperplasia and hypertrophy.
Author’s Answer
“In summary, subcutaneous adipose hypertrophy and hyperplasia occur independently of sex and body fat content and are strongly related to the total adipocyte number in adults. A low generation rate of new adipocytes is associated with adipose hypertrophy, which is linked to low insulin sensitivity and high circulating insulin levels. A high rate is associated with .. adipose hyperplasia.”
My Answer
Fat cells are something no one wants. Yet, they serve an important purpose by storing energy. In the days when our ancestors didn’t farm, being able to preserve energy helped them survive. However, in the modern world, we have food at our fingertips. This isn’t necessarily a good thing because it makes overeating very easy.
Our fat cells can expand and shrink as needed. They can be created anew if we eat too much, but don’t ever really go away. Well, technically, every 8 years 50% of adipocytes are replaced. Scientists haven’t found a way to increase or decrease this rate. Even liposuction won’t get rid of fat cells more so than fat cell volume. So, the best way to prevent future issues with fat cells is to never create them in the first place. If you’re like me it’s too late for that one. Dream bulks made no mention of these issues.
There does seem to be a limit to how many fat cells we can create outside of extreme circumstances. One study assessed total adipocyte number in 687 adult individuals and combined this data with previously reported results on children and adolescents. This is a cross-sectional study (like the current one), which means that participants were only analyzed at a single time-point, not over several weeks. The authors found that fat cell number increases in childhood and adolescence, with the number leveling off and remaining constant in adulthood in both lean and obese individuals. This data indicates that the number of adipocytes might be set by early adulthood with not much change thereafter.
Adipose tissue is an endocrine organ, releasing hormones and adipokines, like leptin. Leptin stimulates energy expenditure and inhibits appetite. Leptin levels are also linked to adipocyte size, such that larger adipocytes produce more leptin than small adipocytes. In addition, smaller adipocytes turnover triglycerides at a slower rate than large adipocytes, resulting in decreased energy expenditure of small adipocytes compared to large adipocytes. When we lose weight we don’t actually lose adipocytes. We just make the cells smaller. This makes it easier to put fat back on after we lose it since the energy needed to create those cells isn’t needed. In a study on participants who had lost a lot of weight (post-obese), there was less circulating leptin than in comparable people who had not lost weight. This translates into them having a higher appetite, which makes the weight harder to keep off.
The pathogenesis of metabolic diseases is significantly influenced by not only how the fat is stored (hypertrophy versus hyperplasia), but also where the fat is stored. People described as metabolically healthy (no metabolic disease), but obese, often have less visceral adipose tissue distribution than obese patients with metabolic disease.
We call studies like this a secondary analysis. Not to say they aren’t worthwhile, they just generally combine a bunch of data from different projects to try to answer a question. This isn’t the best approach, but it can give us some insight into how things work. It’s also how a lot of doctoral students publish papers.
How can we apply this?
This isn’t something you can directly apply to your clients. This is just some cool science you might use to educate them.
What’s next?
Adipocyte biology is somewhat understudied compared to other tissues. For a long time, it was thought that it was mainly a source of energy storage. More recently, it’s been found that our fat cells can communicate with other organs. Now we need to figure out when, how, and what changes effect that communication.